Formulation
Based on data from BioKier’s preclinical and clinical studies and on studies reported in the literature, it was concluded that a sustained-release, colon-targeted oral formulation of butyrate has potential as an oral product for the management of glucose via restoration of gut hormone secretion. Such a formulation was achieved by combining a slow-release tablet core with the proprietary Phloral™ coating which was designed to deliver compounds specifically to the colon.
Video Oral Formulation
Sustained release tablet core
The desired sustained-release capsule core formulation was achieved by testing combinations of various tertiary combinations of excipients (inactive ingredients) and butyrate or L-glutamine in simulated colonic fluid. In initial product BKR-013 L-glutamine was released over 3 h when the capsule core was incubated with stirring in simulated colonic fluid (Figure 7). (Fecal slurry graph to be removed). The goal for current BioKier’s product BKR-017 is to have butyrate released over several hours in the colon environment. Butyrate was released over 10 h when the tablet core was incubated with stirring in simulated colonic fluid (Figure 8)


Phloral™ coating
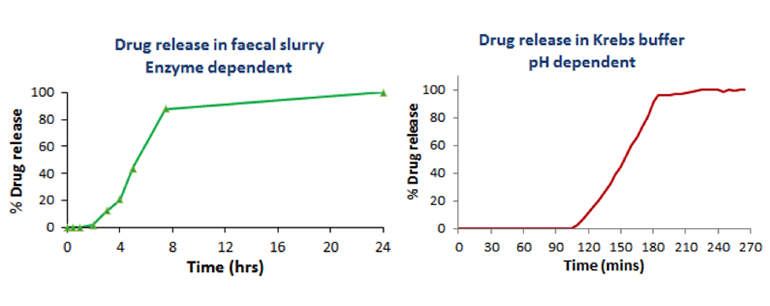
For delivery of the capsule core specifically to the colon, BioKier in-licensed the proprietary colon targeting coating, Phloral™, from University College of London. This unique coating is designed to disintegrate in colonic conditions using a dual mechanism: an enzymatic mechanism based on colonic amylase and a pH (>7.4)-based mechanism. The Phloral™ coating was applied to capsule cores containing a test substance for in vitro testing. Removal of the coating from the capsules under both enzymatic and pH conditions was investigated. Prior to immersion of capsules in each colon-simulated condition, coated capsules were incubated in simulated gastric fluid and simulated intestinal fluid, for 2 h in each fluid, to simulate transit of capsule through human intestinal tract. No release of test substance occurred in simulated gastric or intestinal fluid (not shown). Incubation of coated capsules in fecal slurry, to simulate enzymatic conditions in the colon, and in pH 7.4 Krebs buffer, to simulate colon pH, both resulted in removal of the coating and release of test drug (Figure 9).