BioKier completed a four-week clinical study with BKR-013 (L-glutamine in colon-targeted, sustained-release capsules) in Type 2 diabetes patients, who were already being treated with approved anti-diabetes drugs. This study was supported by an NIH SBIR grant.
Fourteen (14) patients were enrolled in the triple-blind, cross-over study conducted at Pennington Biomedical Research Center in Baton Rouge, LA, with Dr. Kishore Gadde as principal investigator. Each patient underwent two four-week treatment periods separated by a four-week washout period. All patients received both placebo and BKR-013 capsules in random, blinded order. Twelve (12) patients completed both arms of the study and the data were evaluated.
The most significant and encouraging result of the study was an improvement in insulin sensitivity in patients who were insulin-resistant, after the BKR-013 treatment period compared to the placebo treatment. Three patients were insulin-sensitive at the time they were enrolled into the study. Of the nine (9) insulin-resistant patients, 6 demonstrated significant improvement in insulin sensitivity. In those six patients, a lowering of fasting glucose and insulin was also observed. Additionally, in subset of patients there was lowering of fasting and postprandial triglycerides.
HOMA IR (a measure of insulin resistance) improved from 2.86 to 1.67 (n=6) in responders, which compares favorably to 2.75 to 1.27 (n=10) in HOMA-IR in diabetes patients after bariatric surgery (Diabetes 55:2025–2031, 2006).
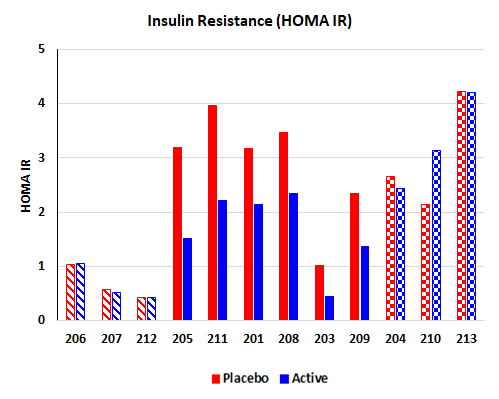
Effects of 4-week treatment with BKR-013 on insulin resistance (HOMA-IR) in individual subjects. Subjects are grouped into three groups: insulin-sensitive (diagonal lines bars), responding insulin-resistant (solid bars), and non-responding insulin-resistant (checkered bars).
It was also observed that the acute effect of L-glutamine in stimulating acute GLP-1 secretion, necessary to maintain long term benefits of this treatment, diminished over the time of treatment. Based on published scientific results, it is hypothesized that this developed because repeated delivery of L-glutamine to the colon resulted in an increased utilization of L-glutamine by colonic bacteria and/or colon wall.
This observation, and the known long-term effects of colonic butyrate on improvement in glucose control, suggests that butyrate would be the preferred active ingredient for chronic regulation of glucose. For this reason, BioKier has developed a colon-targeted, sustained-release butyrate tablet, BKR-017, to be tested in diabetes patients. BioKier has received SBIR funding from NIH to support this study.
